
Insights+: The US FDA New Drug Approvals in July 2023
Shots:
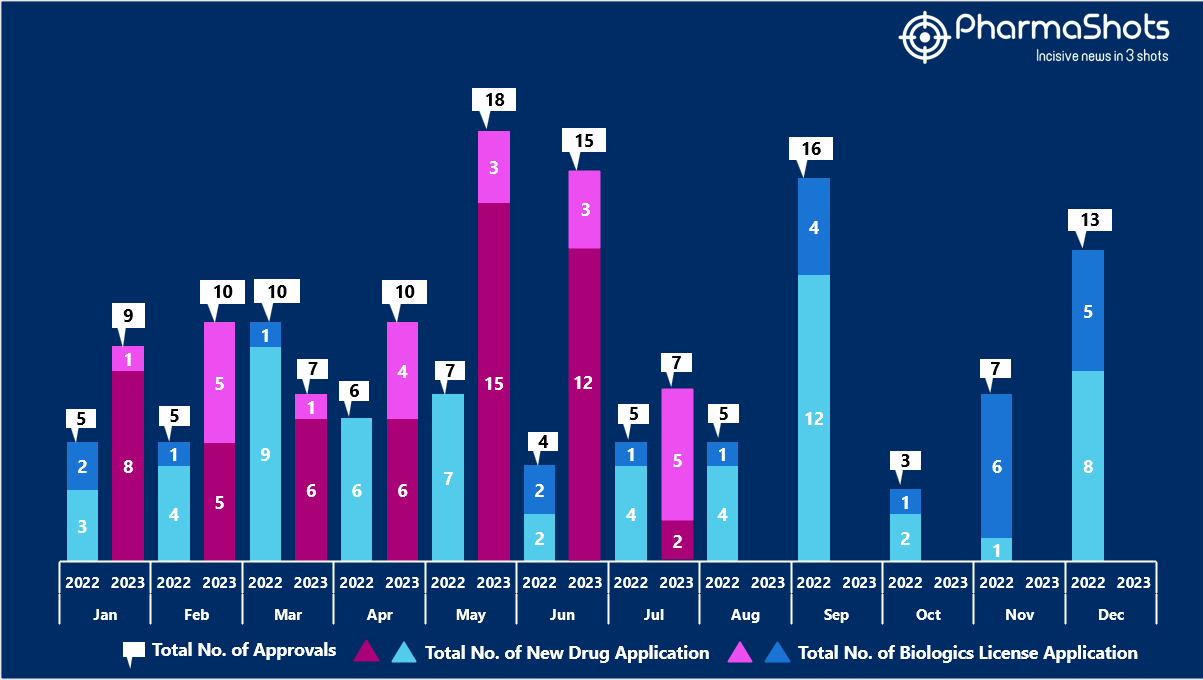
- The US FDA approved 5 NDAs and 2 BLA in July 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 76 novel products in 2023
- In July 2023, the major highlights drugs were Beyfortus (nirsevimab) approval for the prevention of RSV lower respiratory tract disease in infants, Vanflyta for newly diagnosed FLT3-ITD positive acute myeloid leukemia
- PharmaShots has compiled a list of a total of 7 new drugs approved by the US FDA in July 2023
Beyfortus
Active ingredient: nirsevimab Approved: July 19, 2023
Company: AstraZeneca and Sanofi Disease: RSV Lower Respiratory Tract Disease
- The US FDA has approved Beyfortus for RSV LRTD in newborns & infants born during or entering their first RSV season, and for children ~24mos. who remain vulnerable to sev. RSV disease through the 2nd RSV season. The therapy is expected to be available in the 2023-2024 RSV season
- The approval was based on the extensive clinical development program incl. 3 late-stage trials evaluating Beyfortus. The results showed that a single dose of Beyfortus demonstrated consistent efficacy against RSV LRTD through 5mos. across all clinical EPs, was well tolerated with a favorable safety profile consistent & overall rates of AEs were comparable b/w Beyfortus & PBO
- The therapy was approved in the EU, Great Britain & Canada while regulatory applications are under review in China, Japan & multiple other countries
Vanflyta
Active ingredient: quizartinib Approved: July 21, 2023
Company: Daiichi Sankyo Disease: Acute Myeloid Leukemia
- The US FDA has approved Vanflyta in combination with standard cytarabine and anthracycline induction and cytarabine consolidation & as maintenance monotx., following consolidation CT for adult patients with newly diagnosed AML i.e., FLT3-ITD+
- The approval was based on the P-III trial (QuANTUM-First) results published in The Lancet evaluating Vanflyta + standard induction and consolidation therapy incl. HSCT & as maintenance monotx. in a ratio (1:1) in 539 patients aged 18-75yrs. at 193 study sites across Asia, the EU, North & South America, and Oceania
- The results showed a 22% reduction in risk of death, CR rates were similar b/w both arms, and the median duration of CR (38.6 vs 12.4mos.) in PBO + standard CT alone. The therapy is expected to be available by prescription in the US in the coming weeks
NexGard PLUS
Active ingredient: afoxolaner, moxidectin, and pyrantel Approved: July 21, 2023
Company: Boehringer Ingelheim Disease: Internal and External Parasites
- The US FDA has approved afoxolaner, moxidectin & pyrantel chewable tablets (NexGard PLUS), a new one-and-done monthly combination product for canine protection from fleas, ticks, heartworm disease, roundworms & hookworms
- Multiple clinical studies showed that NexGard PLUS was safe & effective, ≥99.8% effective 24hrs. in killing adult fleas fast after weekly infestations for a full month after treatment, 100% effective in preventing heartworm disease in dogs, safe for puppies as young as 8wks. & in P-gp-deficient (MDR1-mutant) avermectin-sensitive dogs
- Boehringer Ingelheim’s beef-flavored afoxolaner, moxidectin & pyrantel chewable tablets are expected to be available for veterinary clinics on July 2023
Ycanth
Active ingredient: cantharidin Approved: July 24, 2023
Company: Verrica Pharmaceuticals Disease: Molluscum Contagiosum
- The US FDA has approved Ycanth (cantharidin) topical solution for adult & pediatric patients aged ≥2yrs. with molluscum contagiosum. The approval was based on 2 P-III trials (CAMP-1 & 2) that evaluated VP-102 (Ycanth) vs PBO in patients aged ≥2yrs.
- Both trial results showed that patients treated with VP-102 met its 1EPs of complete clearance of all treatable molluscum lesions, 46% vs 18% achieved complete clearance of molluscum lesions in the (CAMP-1) trial while 54% vs 13% in the (CAMP-2) trial
- In an additional post-hoc analysis, complete clearance of all lesions was higher in the VP-102 group vs vehicle across all body regions. Ycanth, a drug-device combination product administered by a healthcare professional is expected to be available in Sept 2023
Xdemvy
Active ingredient: lotilaner Approved: July 27, 2023
Company: Tarsus Pharmaceuticals Disease: Demodex Blepharitis
- The US FDA has approved Xdemvy, the first and only US FDA-approved treatment to directly target Demodex mites for the treatment of Demodex blepharitis. Xdemvy is expected to be available by prescription at the end of Aug 2023
- The approval was based on two studies (Saturn-1 & 2) evaluating Xdemvy vs vehicle in a ratio (1:1) in 833 patients which showed an improvement in eyelids (reduction of collarettes, the pathognomonic sign of the disease, to no more than 2 collarettes per upper lid) in each study by Day 43 with some patients showed improvement as early as 2wks.
- The EPs of mite eradication & erythema cure showed an improvement at Day 43 across both studies, and were safe & well tolerated while other ocular adverse reactions were reported in ≤2% of patients
6. BioMarin’s Roctavian Receives the US FDA’s Approval for the Treatment of Severe Hemophilia A
Roctavian
Active ingredient: valoctocogene roxaparvovec Approved: July 30, 2023
Company: BioMarin Disease: Hemophilia A
- The approval was based on the P-III (GENEr8-1) study evaluating Roctavian in patients (n=134) with severe hemophilia A. For 112 patients, baseline ABR data were collected during 6mos. on FVIII prophylaxis before receiving Roctavian & for 22 patients, baseline ABR was collected retrospectively
- The results showed that in 112 patients, a mean ABR reduction of 52% was reported after receiving Roctavian by the end of follow-up (2.6 bleeds/year) vs baseline ABR while receiving routine FVIII prophylaxis (5.4 bleeds/year) & rate of spontaneous bleeds & joint bleeds was also reduced with Roctavian (0.5 & 0.6 vs 2.3 &3.1 bleeds/year)
- BioMarin expects to continue to monitor the long-term effects of the treatment with a follow-up of 15yrs.
Jemperli
Active ingredient: dostarlimab Approved: July 31, 2023
Company: GSK Disease: Endometrial Cancer
- The approval of Jemperli (dostarlimab-gxly) + carboplatin + paclitaxel, followed by Jemperli as monotx. for the treatment of adult patients with dMMR primary advanced or recurrent endometrial cancer
- This approval is based on the interim analysis results from Part 1 of the P-III study (RUBY), which demonstrated a median duration of follow-up of ≥25mos. A significant and clinically meaningful benefit in PFS and a 71% reduction in the risk of disease progression or death. The trial continues to evaluate OS
- Consistent safety and tolerability were seen over the monotherapies. The most common TEAEs were ≥20%. Also, the data were presented at ESMO and SGO 2023 and published in The New England Journal of Medicine
Related Post: Insights+: The US FDA New Drug Approvals in June 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














